Influenza & COVID-19

Influenza
Every year we eagerly await the 'flu letter' so we know what we are doing in the coming season.
The ‘flu letter’ with the intentions for the 25/26 season arrived 13th February 2025 (and advice for 26/27 is already trickling though)
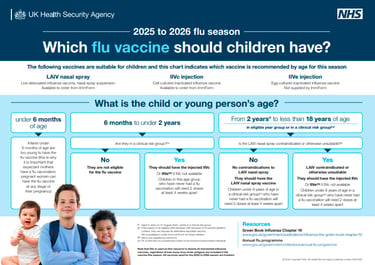
We began the 25/26 vaccine clinics from 1st Sept for the kids and pregnant ladies, and 1st Oct on the adult and clinical risk groups 18+. This season and 24/25 are the only years we have staggered the starts and this is due to quicker waning immunity on older adults than in youngsters. An extra month to wait means the vaccine protection will carry through to the end of flu season.
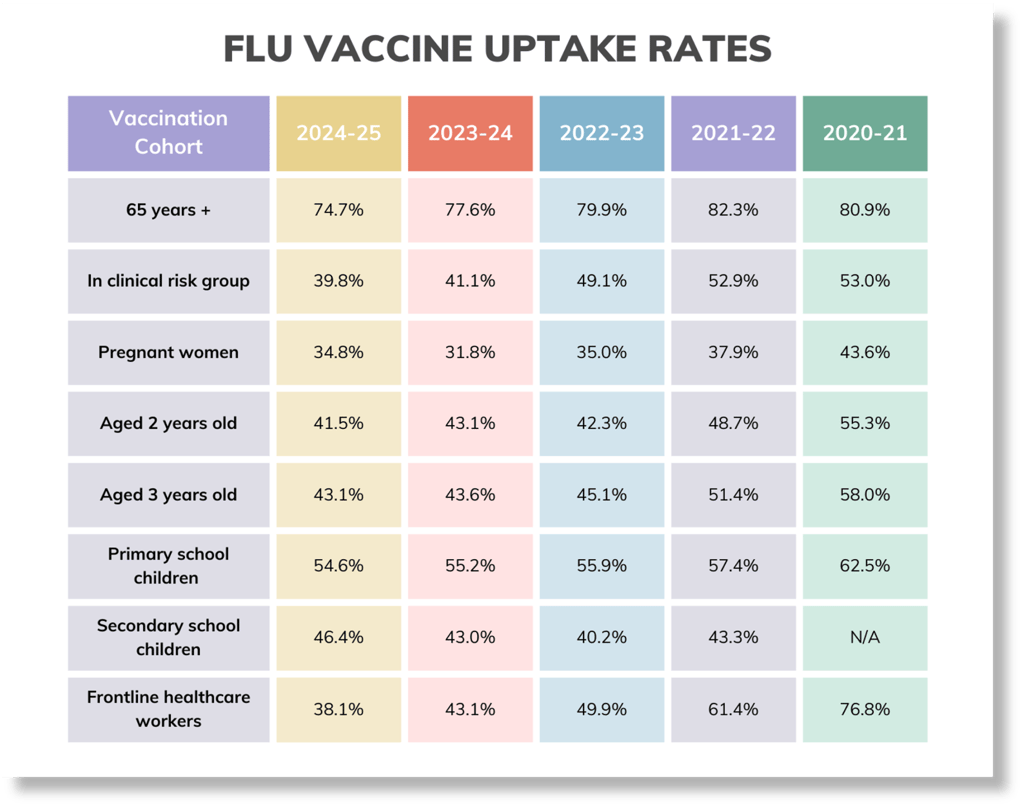
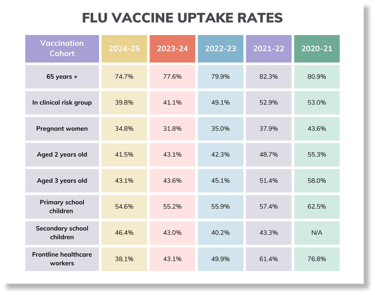
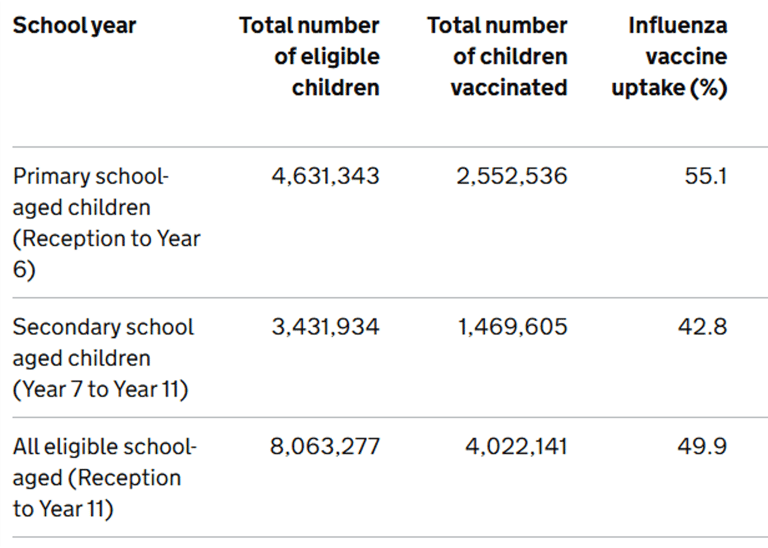
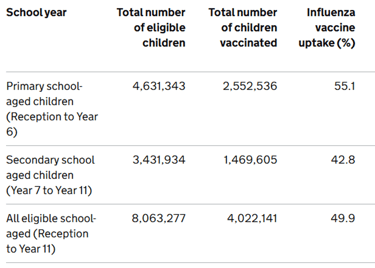
It's a massive undertaking to deliver the flu programme. And vaccine uptake across all groups has consistently droped since COVID-19 times. As of 23rd October we had vaccinated over 10.4 million (10,436,395) people against flu and almost three million (2,987,313) people against COVID. Well done all!! As of 8th Jan 2026 over 18.6 million eligible people were vaccinated. With flu still circulating please continue to do all you can to encourage eligible people to have their vaccine.
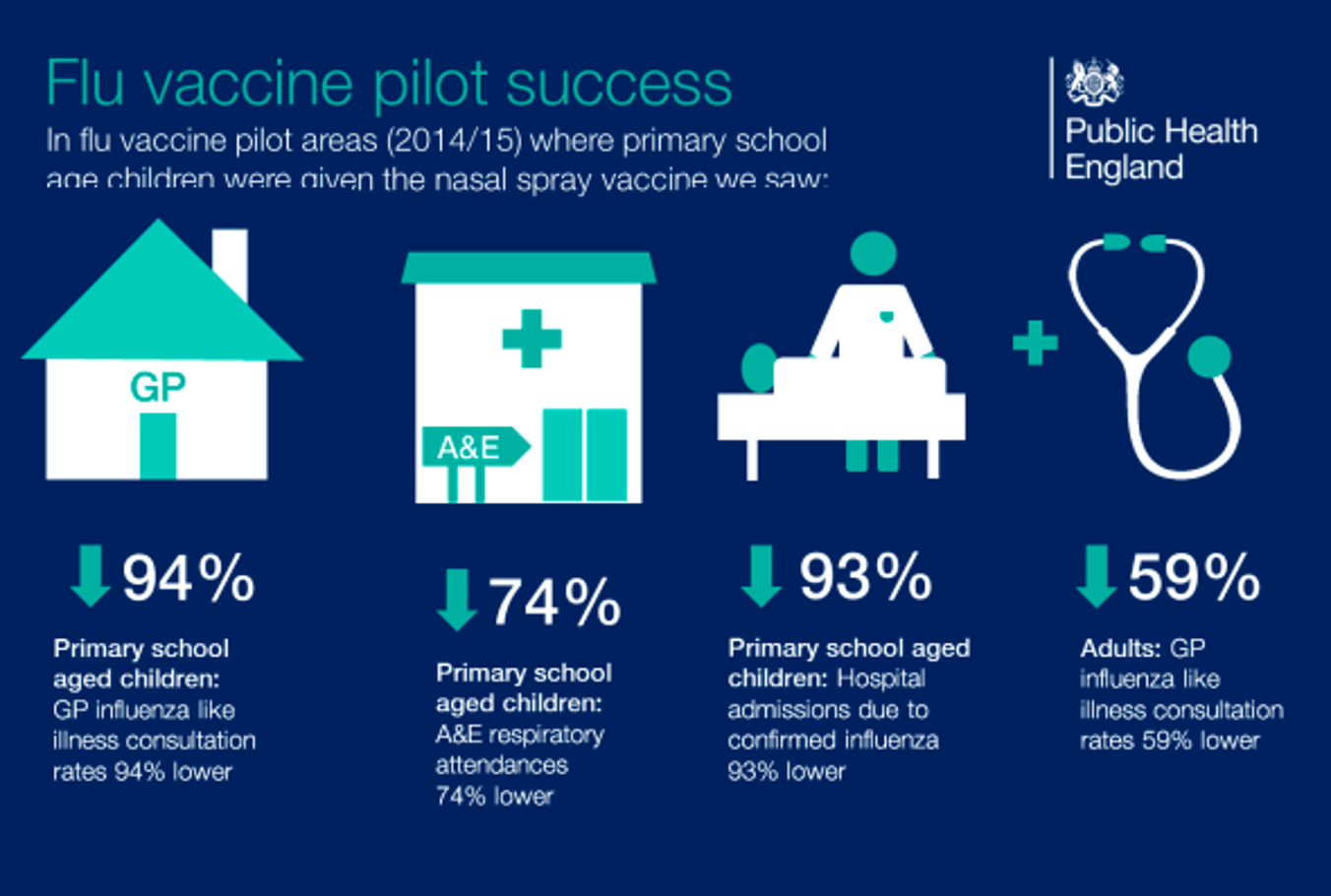
UKHSA published modelling estimates which suggested that flu vaccination had prevented approximately 100,000 hospitalisations in the 24/25 season in England
We hit the 25/26 flu season five weeks earlier than usual. Australia experienced a severe winter; France and Japan also reported very high activity. There was a mutated A strain in the summer which is always a risk when deciding on vaccine strains so far in advance. Hence, 25/26 is a VERY challenging season. Fortunately the 25/26 vaccines have maintained their effectiveness.
QUICK CHANGES-FROM-LAST-YEAR NEWS!
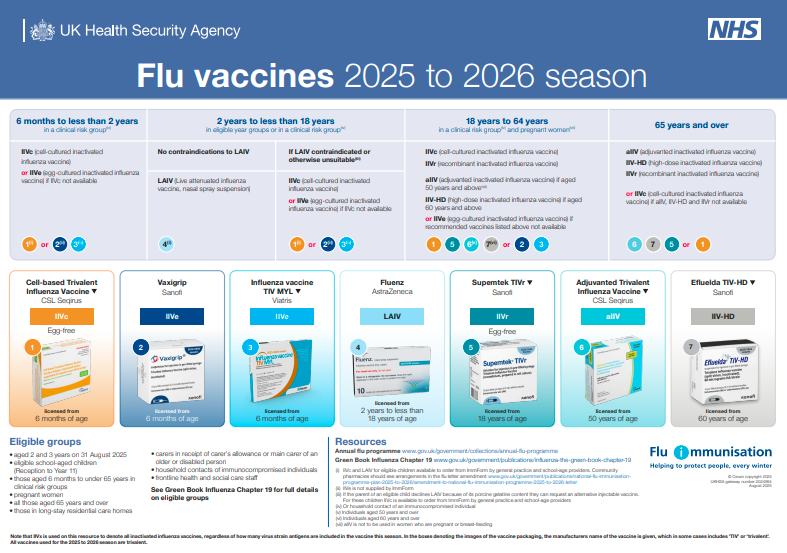
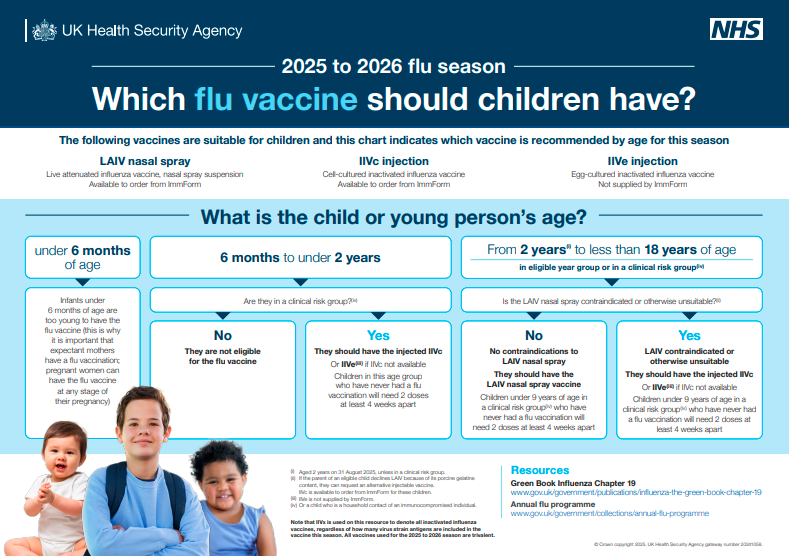
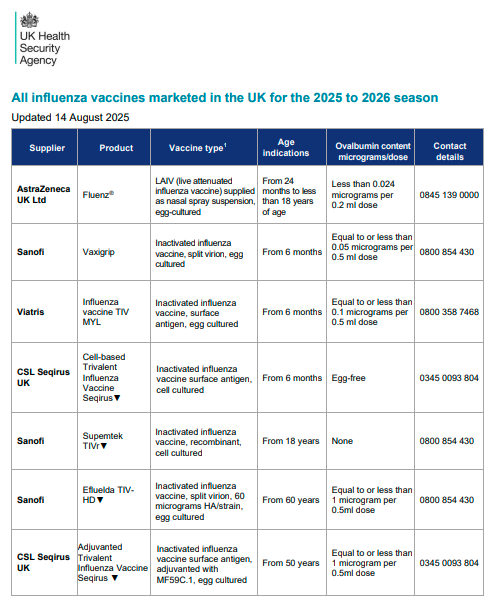
Previous years have seen quadrivalent vaccines (4 strains) but 25/26 said goodbye to the Yamagata B strain (due to not circulating for a while) and all UK vaccines are now trivalent.
aTIV now licenced from 50 years (TIVHD (60+) could follow this pattern for next year too).
TIVr made a return in 25/26!
LAIV now includes PGD provision for up to 25y in SEN schools.
Community pharmacy getting involved with 2/3y olds.

• LAIV is different from other flu vaccines – it is a live attenuated nasal vaccine and must not be injected
• Do not attempt to attach a needle
• Fluenz can be administered at the same time as, or at any interval from other vaccines including live vaccines
• Patient should breathe normally - no need to actively inhale or sniff
• The vaccine is rapidly absorbed so no need to repeat either half of dose if patient sneezes, blows their nose or their nose drips following administration
Administration of Nasal Flu Vaccine

IM administration
Note she doesn’t actually insert the needle in this demo (don’t forget to put it in!!!)


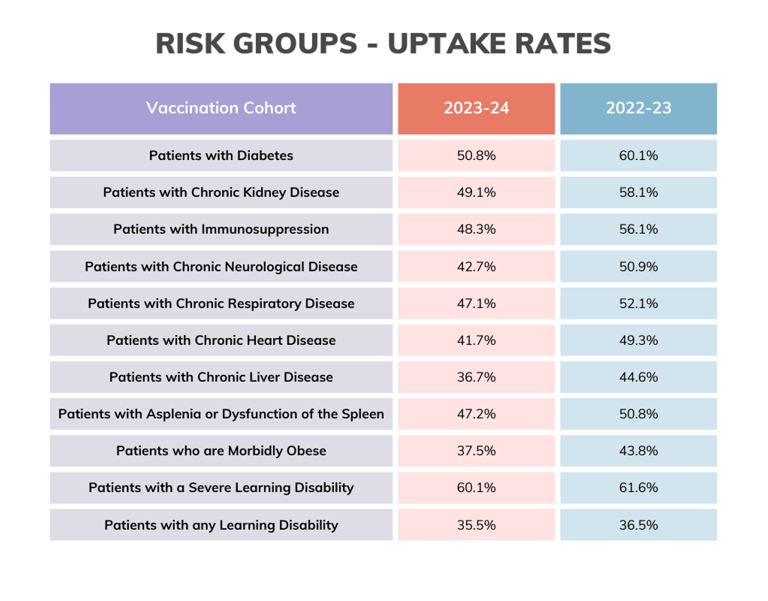
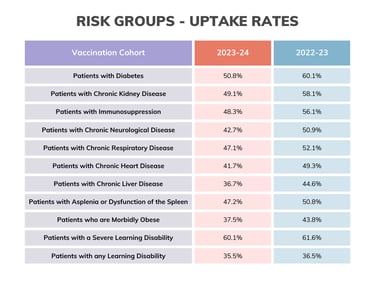
Results: "Access was NOT the primary issue underlying suboptimal vaccine uptake among participants in clinical risk groups, who instead cited low-risk perceptions of influenza infection and deficits of information about the relevance of vaccination for their condition management. Healthcare providers in non-primary care settings rarely discussed or recommended influenza vaccination across patient pathways, despite being able to address the concerns raised by participants in clinical risk groups." (I.E. vaccine uptake is not just about putting on the late night and weekend clinics! People may well make more efforts to show up if they knew WHY they should)

Reflection points:
Do you know your local uptake figures?
In YOUR clinic, which are the most difficult groups to reach? Why?
Do you see similar results to the national figures?
What other vaccines might these groups be missing out on?

Ovalbumin content
LESS THAN 0.12MCG/ML = SAFE TO VACCINATE
(equivalent to <0.06mcg for 0.5ml dose)
Porcine Gelatine Content
Fluenz Tetra contains a small amount of highly purified porcine gelatine. This vaccine is the most effective option for children aged 2 and older but there is the injectable alternative if required.
Think about how these conversations are had....
Other queries about vaccine contents... Dogs, sharks and moths?

COVID-19
At least 15–20 million lives saved globally in the first year alone—and many more since—according to the modelling.
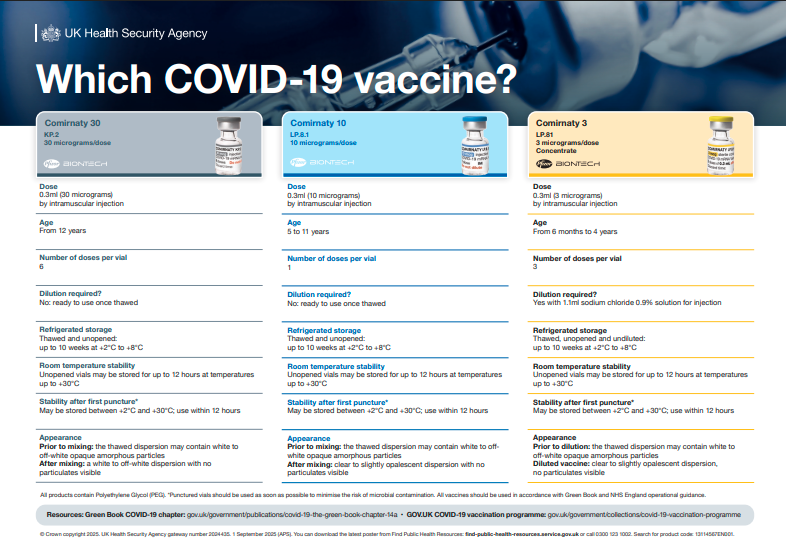
Autumn 2025 booster programme underway 1st Oct-31st Jan, Check out the new variant vaccines (and the helpful new poster). Plenty of ongoing research (boosters & new variants). Hundreds of vaccines in development.
Eligibility adapting to context. Eligibility is narrowing - now 75+, care home residents and IC 6m+. Check out this headline: Pharmacies facing angry patients over Covid jab confusion.
Could COVID-19 move to an all year round prog? JCVI minutes from June indicate it's up for consideration.
Vaccines evolving – bivalent options, new variants, nasal options? Combined vaccines? Where is the 2 in 1 COVID and flu jab up to? Could this be with us in 2026?
Legal changes have lead to some confusion and misunderstandings with other vaccines - check your legal mechanisms!
Pandemic disruption has left much catching up to do with other vaccines